What Is Plasma and Why Is It Needed?
Plasma-derived medicinal products (PDMPs) are essential, life-saving treatments for people with rare, chronic, and immune-related conditions. These medicines cannot be manufactured synthetically - they rely entirely on donated human plasma, a unique and irreplaceable resource.
While these therapies transform the lives of patients, it is plasma donors who make them possible. Donor health and safety is a public health priority, and the entire donation process is designed to be medically safe, ethically sound, and scientifically monitored.
Plasma is the yellowish liquid portion of blood that carries vital proteins and antibodies. It is used to produce more than 20 different PDMPs to treat diseases such as:
Primary immunodeficiency (PID)
Hemophilia
Alpha-1 antitrypsin deficiency
Guillain-Barré syndrome (GBS)
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Some of these conditions have no alternative treatment. A single patient's yearly therapy may require:
· ~130 plasma donations for PID
· ~360 donations for CIDP
· ~900 for Alpha-1 antitrypsin deficiency
· ~1,200 for hemophilia
This is why repeat plasma donations are needed, and why protecting donor well-being is essential for building a sustainable and ethical plasma supply.
Source Plasma vs. Recovered Plasma
There are two types of plasma used in the manufacture of plasma-derived medicinal products (PDMPs): source plasma and recovered plasma.
Source plasma is collected through a process called plasmapheresis, in which plasma is separated from the donor’s blood and the remaining components, such as red blood cells, are returned to the donor. This procedure typically takes between 60 and 90 minutes and is specifically designed for the purpose of plasma collection. As a result, source plasma represents the majority of plasma used in the production of PDMPs.
In contrast, recovered plasma is obtained from whole blood donations. After a standard blood donation, the blood is separated into its various components, and the plasma portion is recovered for further use. However, recovered plasma makes up a smaller share of the overall supply due to the lower volume obtained and less frequent donation rates.
While both types of plasma are suitable for PDMP manufacturing, source plasma is generally preferred because of its higher availability, efficiency, and the rigorous, purpose-built safety protocols involved in its collection.
Introduce your brand
Donor Health and Safety: A Shared Responsibility
Plasma donation is proven to be as safe as donating blood. A well-defined, multi-step process ensures that only healthy individuals donate, and that each step of donation protects both the donor and the recipient.
1. Donor Qualification and Screening
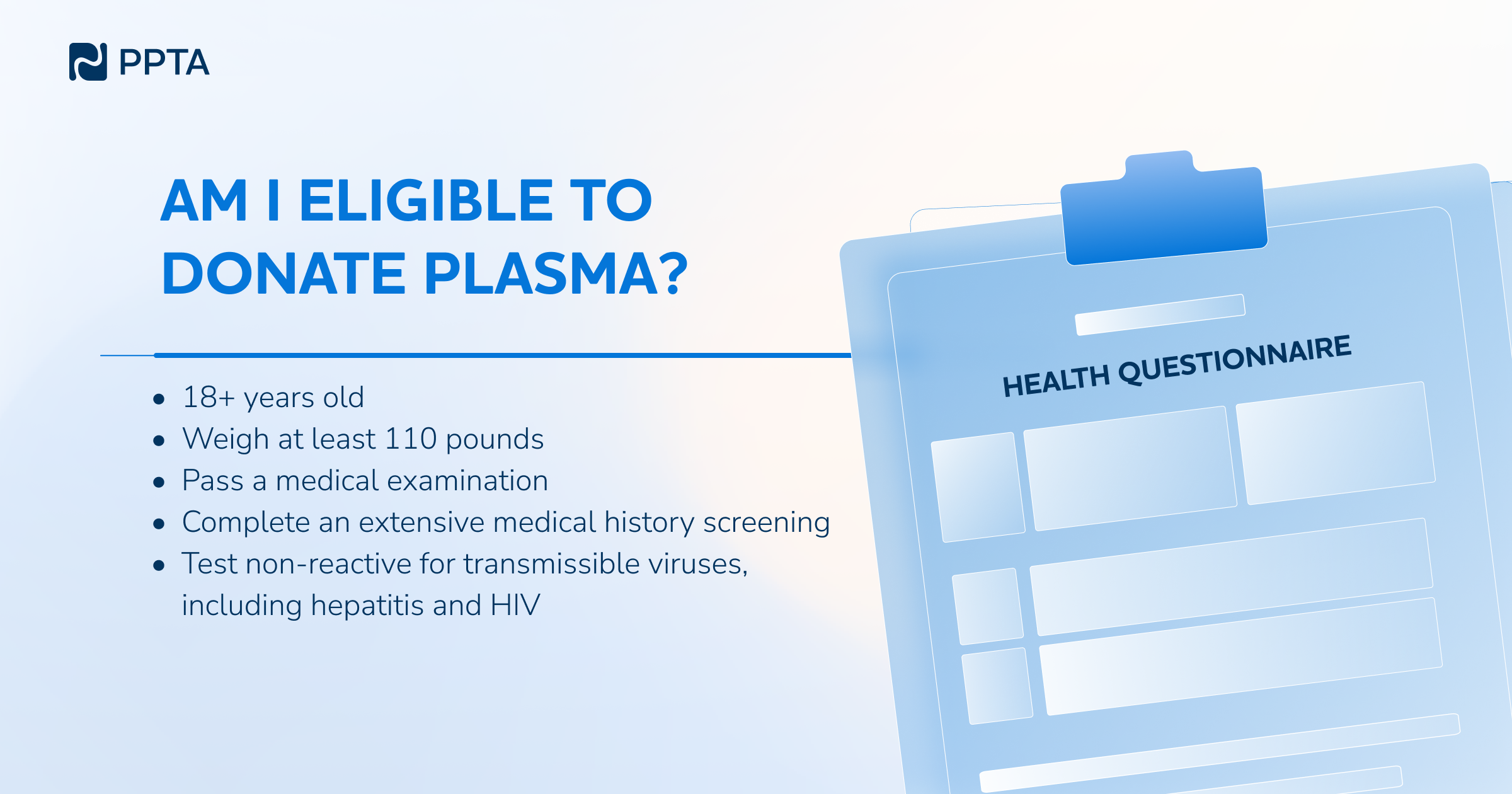
Before every donation, donors must meet strict health criteria set by national health authorities.
Eligibility criteria:
· At least 18 years old
· Minimum 50 kg (EU) / 110 lbs (U.S.)
· In good general health
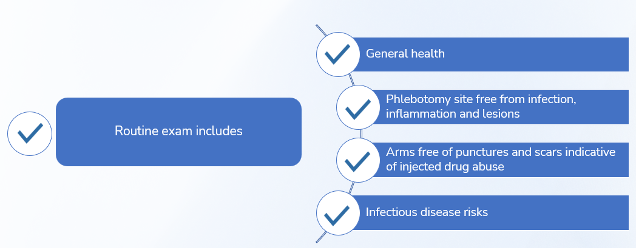
Health checks at every visit:
· Blood tests for protein and immunoglobulin levels
· Blood pressure, weight, pulse
· Skin examination at the needle site
· Review of medical history
If any issues are detected, donation is postponed to protect the donor’s well-being. Plasma is also tested for infectious diseases, including HIV, Hepatitis B and C, and more.
Donors are also advised on how to prepare for a smooth donation:
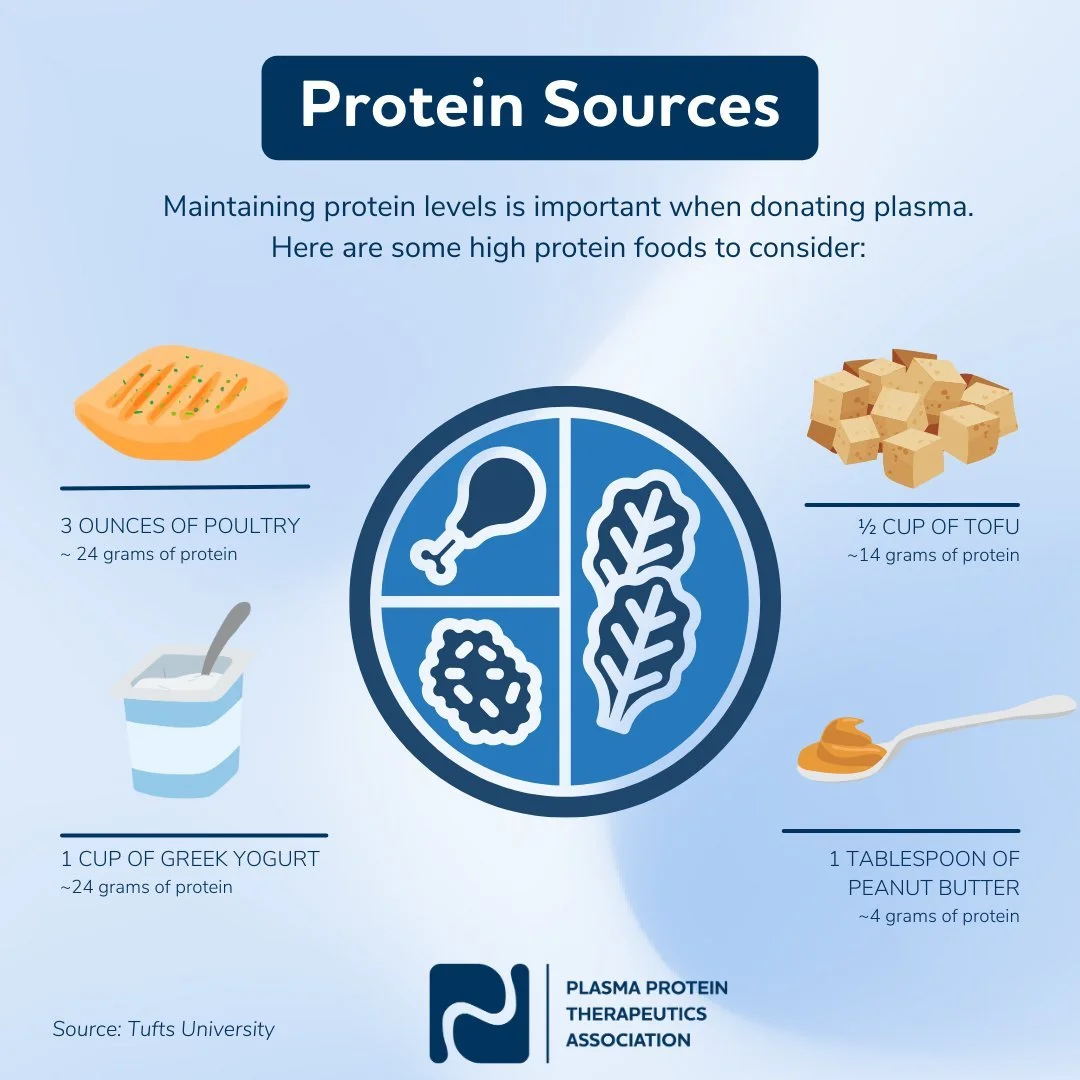
· Include protein in the diet
· Eat a balanced meal
· Stay hydrated
2. The Donation Process: Safe and Monitored
The donation process – plasmapheresis - takes about 60-90 minutes. It involves drawing blood, separating the plasma, and returning red blood cells and other blood components to the donor.
This process is supervised by trained healthcare staff. All equipment is sterile and single-use. First-time donors are monitored for an extra 15-20 minutes after donation. Donors are also offered IV fluids to support recovery.
99.84% of all plasma donations are completed safely, with most side effects being mild and temporary (e.g., dizziness or bruising).
3. Safe Repeat Donations
Because plasma regenerates faster than red blood cells, donors can give plasma more frequently. Donation frequency is regulated by national health authorities.
National limits vary from 2 to 104 donations per year in reality 50% donate fewer than 10 times annually
All donations are tracked and monitored for safety. PPTA’s established donor adverse event recording standard assures this.
Scientific studies involving over 9,000 donors confirm that regular plasma donation does not harm long-term health when done according to the rules and regulations.
How Is Plasma Safety Assured?
From donation to final product, each drop of plasma goes through rigorous safety processes to protect everyone involved.
Key steps include:
Screening of donors and donations at multiple points
Testing of individual and pooled plasma units
Viral inactivation and removal during manufacturing (heat, solvent-detergents, nanofiltration, pH)
Traceability of every donation
Continuous innovation in safety and donor monitoring systems
There have been no reports of viral transmission from plasma-derived therapies for over 25 years, even with emerging viruses like COVID-19 and Zika.
A Shared Commitment to Public Health
Plasma donation plays a critical role in public health by enabling access to PDMPs for people with serious and often lifelong conditions. Meeting the growing need for these treatments requires more than a safe and efficient collection system, it depends on a broad and sustained commitment across the health ecosystem.
At the center of this system are the donors. Their willingness to give plasma supports patients who depend on these therapies to manage their health and maintain their quality of life. Protecting donor health is not just a procedural requirement - it’s a public health responsibility. Donation centers must provide a safe, well-regulated environment, and healthcare authorities must ensure that guidelines are clear, evidence-based, and consistently applied.
Plasma donation also benefits from transparency and collaboration. Ongoing research, safety monitoring, and data sharing contribute to maintaining public confidence and continuously improving practices. When patients, donors, healthcare providers, and policymakers are informed and engaged, the result is a more resilient and ethical system, one that respects the people at its core and delivers real-world health benefits.
In short, plasma donation is a shared effort. It relies on individuals who donate, professionals who safeguard the process, and a wider public that values and supports this essential part of healthcare.
Acknowledgement
This section was developed with kind support of the Plasma Protein Therapeutics Association (PPTA), which provided insights into current industry standards and safety practices. The information reflects broadly applied approaches across the sector, rather than those of any single plasma collector or manufacturer.